Medical terminology is used throughout this analysis, which lets you conduct an intelligent conversation with your therapy provider. The first use of a medical term is highlighted in boldface text. A definition either precedes or follows the term’s first use. A section containing a complete glossary will be provided at the end of the last article in this series. To better understand this new direction in medical science, the first term you need to master is “orthobiologics.”
Orthobiologics are substances that orthopedic surgeons use to help injuries heal more quickly. This rapidly evolving science includes applying cell-based therapy, including bone marrow aspirate concentrate (BMAC), hyaluronic acid, platelet-rich plasma (PRP), and other yet-to-be-discovered biologic substances.
Orthobiologics is just one aspect of the new field of “regenerative medicine” — which, as the name implies, specializes in regenerating or replacing tissue or organs to help patients regain abilities lost due to disease, trauma, or congenital issues, or weakened due to aging. Hormone replacement therapy (HRT), which relies on estrogen, HGH, and testosterone to achieve similar objectives, represents the other branch of regenerative medicine.
History of Stem Cells
In 1908, Russian histologist Alexander Maksimov first proposed the term “stem cell” for scientific use.
Stem cells have the ability, under certain conditions, to self-renew by dividing and developing into specialized cell types present in specific tissues or organs — a process scientists refer to as “differentiation.” Cells that have the capacity to differentiate into many types of cells are called “multipotent stem cells.”
In summary, the definition of a stem cell is a cell that can:
- Self-renew – Possesses the ability to create more copies of itself.
- Differentiation – An ability to divide to create other cell types that mature into cells with specific body functions.
The first successful use of stem-cell restoration happened in 1968 when a bone marrow transplant was performed in Minnesota on two siblings who each suffered from severe immunodeficiency.
Ten years later, in 1978, stem cells were discovered in umbilical cord blood. In 1981, came another breakthrough, the first in-vitro stem cell line created from mice. It took another 14 years to derive the first embryonic stem cell from a primate, a rhesus monkey.
That was followed in 1997 by the first stem-cell cloning of a sheep. The lamb, called Dolly, was the first mammal cloned from an adult cell, using a process called somatic cell nuclear transfer. This process involves transferring a cell nucleus from an adult cell to an unfertilized, developing egg cell that had its nucleus removed. Dolly was cloned by Ian Wilmut, Keith Campbell, and colleagues at the Roslin Institute, part of the University of Edinburgh, Scotland.
 Roslin Institute Professor Ian Wilmut, whose team created the world’s first mammal cloned from an adult cell, says stem cell research might still lag 20 years behind if Dolly the Sheep had never been born in 1997.
Roslin Institute Professor Ian Wilmut, whose team created the world’s first mammal cloned from an adult cell, says stem cell research might still lag 20 years behind if Dolly the Sheep had never been born in 1997.
Image courtesy: Alamy Stock Photo.
In 1998, James Thomson and co-workers at the University of Wisconsin–Madison isolated cells from the inner cell mass of early embryos and derived the first human embryonic stem cells. In more than a dozen landmark papers published in 1999 and 2000, scientists reported that bone marrow cells could produce nerve or liver cells, and that brain cells could also yield other cell types. Science proclaimed stem cell research “Breakthrough of the Year” in 1999.
In a little more than two decades, the field of stem cell research had advanced by quantum leaps, exciting the global scientific community. That excitement was tempered, however, by President George W. Bush who in 2001 issued an executive order banning federal funding for new sources of stem cells developed from preimplantation human embryos. His action effectively froze U.S. stem cell research and put a temporary halt to rapid innovation.
In 2006, a Kyoto University scientist, Shinya Yamanaka, and a graduate student, Kazutoshi Takahashi, revived the field by creating a way to induce any adult cell, such as a skin cell, to revert to its earliest “pluripotent” stage — an iPS cell.
In its pluripotent stage, a cell can generate nearly all cell types that make up the human body, much like embryonic stem cells. These cells can become any type of cell, from a heart muscle cell to a neuron. The process of maturing stem cells from a pluripotent state to an adult tissue type is called differentiation.
In 2017, Yamanaka added this cautionary note: “iPS cells are only 10 years old. The research takes time. That’s what everybody needs to understand.”
Now that it’s 15 years since the discovery of the iPS cell, where do we stand, Mr. Shinya Yamanaka?
What Type of Stem Cells Are There?
Regenerative medicine typically relies on six major types of stem cells:
- Embryonic stem cells (ESCs) – A normally developing embryo will contain about six to 10 cells three days after fertilization. By the fifth or sixth day, the fertilized egg becomes a blastocyst — a rapidly dividing ball of cells. The use of ESCs is illegal in the U.S. due to practical and ethical concerns surrounding the requisite destruction of human embryos to harvest ESCs, even though embryos utilized come from four- to five-day-old embryos left over from in vitro fertilization (IVF) procedures.
- Induced pluripotent stem cells (iPS) – Innovative cellular reprogramming techniques, pioneered by Shinya Yamanaka et al., have raised the possibility of generating induced pluripotent stem cells from mature cells, such as skin, fat cells, and white blood cells. In 2019, three Hebrew University scientists transformed skin cells into the three major stem cell types that comprise early-stage embryos.
- Adult stem cells – Adult multipotent stem cells can be found throughout adult tissues and have the ability to form multiple, different cell types within a specific tissue type (differentiation).
- Mesenchymal stem cells (MSCs) – MSCs are found in bone marrow and are also multipotent — able to form a wide variety of tissues, such as fat, bone marrow, vessels, cartilage, and muscle.
- Adipose-derived stem cells (ASCs) – Adipose stem cells are found in fat (adipose) tissue and are a useful cell source given their abundance in subcutaneous (under-skin) adipose tissue and ease of harvesting via liposuction.
- Very Small Embryonic Like Stem Cells (VSELs) – In 2008, Marius Ratajczak et al. published a paper describing VSELs as “a population of epiblast-derived (outermost embryo layer) cells that are deposited during early tissue/organ development and play an important role in the creation of tissue-specific stem cells. The paper speculates that VSELs deposited in bone marrow could lead to long-term repopulating of human stem cells.
The above classification requires a further explanation of potency levels possessed by each stem cell:
- Totipotent – Totipotent cells can form all the cell types in a body, including extraembryonic (placental and umbilical cord) cells. This gives totipotent cells the ability to give rise to a complete organism. Embryonic cells within the first couple of cell divisions after fertilization are the only cells that are totipotent.
- Pluripotent – Pluripotent cells can generate all of the cell types that make up the body. After embryonic stem cells lose their totipotency, they become pluripotent, as are, of course, iPS cells.
- Multipotent – Multipotent cells can develop into more than one cell type, but are more limited than pluripotent cells. Adult stem cells, mesenchymal stem cells found in bone marrow, and umbilical cord blood stem cells are considered multipotent.
We can thank today’s widespread use of bone-marrow stem cells to the University of Toronto cellular biologist Ernest McCulloch and his colleague, Dr. James Till, who in 1963 discovered the presence of self-renewing cells in mouse bone marrow.
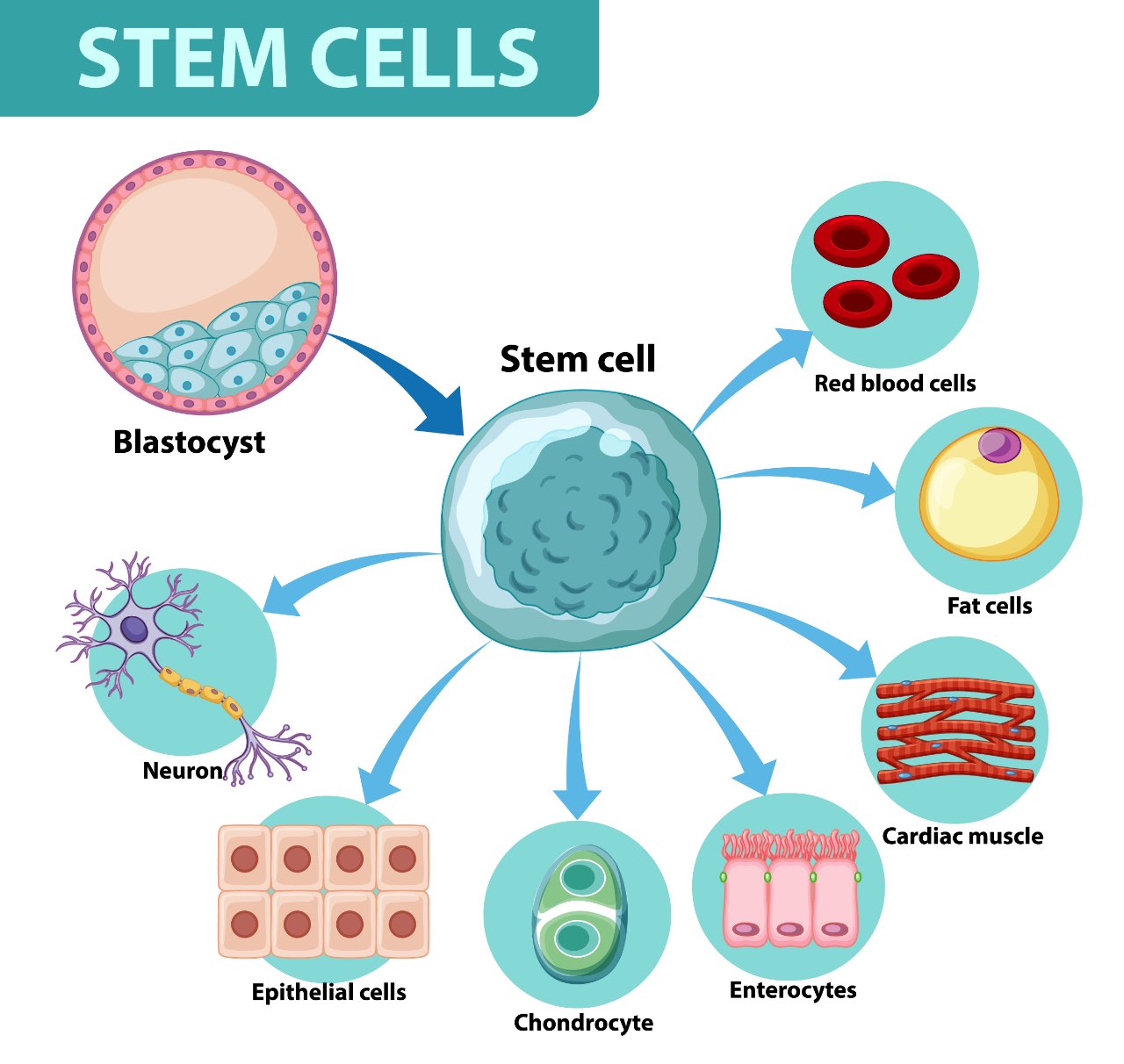
A multipotent cell, such as a mesenchymal stem cell, can differentiate into a host of cells, including a red blood cell, fat cell, cardiac muscle, intestinal cell (enterocyte), cartilage cell (chondrocyte), skin cell (epithelial cell), and nerve cell (neuron).
Blood cells vector created by brgfx.
Who Discovered Stem Cell Treatment?
In 2005, Dr. Christopher Centeno, a pain medicine specialist with no background in stem-cell research came across a study that hinted that stem cells could be used to treat spinal conditions in rabbits. That year, Centeno and Dr. John Schultz established Broomfield, Colo.-based Regenexx, one of the first clinics in the world to use stem cells to treat orthopedic injuries.
In Feb. 2020, Regenexx issued a press release stating that “to date, more than 40,000 patients have been treated and the organization has performed more than 90,000 procedures.” In May 2019, Regenexx told The New York Times that 70,000 of the 90,000 procedures performed involved only platelets (PRP).
In a recent blog post, Centeno reports that Regenexx has treated about 8,000 knee arthritis patients.
Of course, there were many other players in the stem cell treatment field before Centeno. One of the most celebrated ones was Paris, France-based Phillipe Hernigou. In 1989, Hernigou treated his first patient with mesenchymal stem cells (MSCs) at Henri-Mondor University Hospital for osteonecrosis of the femoral head (ONFH), also known as avascular necrosis.
Avascular necrosis (AVN) of the hip, also referred to as aseptic necrosis, is a condition where the blood supply to the ball of the hip joint (femoral head) is lost, causing the bone to die.
In 1993, Hernigou, together with Frederik Beaujean began a study of 189 hips (116 patients) who had mesenchymal stem cells (in the form of concentrated iliac crest bone marrow) injected through core decompression (surgical drilling into the area of dead bone near the joint).
The first mid-term results, reported in 2002, found that only nine of 145 hips required total hip arthroplasty (THA), or total hip replacement.
What Does a Stem Cell Treatment Involve?
So what is a stem cell treatment exactly? How are stem cells obtained? Does stem cell therapy use your own stem cells? How is stem cell treatment done? These are all top-of-mind questions typically asked by people exploring stem cell treatments.
Since Regenexx was the first medical practice to offer stem cell therapy in the U.S., dating as far back as 2005, the company’s procedures represent good examples of stem cell treatments. Regenexx offers two types of stem cell treatments, which do a good job of explaining stem cell therapy:
Regenexx-SD
Regenexx-SD is an orthopedic treatment during which stem cells are harvested and re-injected on the same day. The complete treatment, however, takes place over a few days because it’s preceded by Prolotherapy, which involves injecting a sugar or saline substance into a sore joint or muscle, where it acts as an irritant. It’s believed that the body recognizes the irritant and sends immune cells and other chemicals to the area, starting the body’s natural healing process. A few days later, bone marrow is “aspirated” — removed by injection from your iliac crest — the largest of three bones that make up the pelvis. This bone marrow aspirate (BMA) is typically centrifuged at 2,400 rpm for 10 minutes, which results in bone marrow aspirate concentrate (BMAC).
Regenexx-C
Regenexx performed this procedure in the U.S. until July 2008, when Centeno received an FDA warning letter that said, in part:
“Based on information posted on your website, mesenchymal stem cells utilized in your Regenexx C procedure are drawn from a patient’s bone marrow, sent to a lab, isolated, and then grown using growth factors drawn from the patient’s blood before being inserted back into the patient. These cells are considered drugs because the therapeutic claims shown on your website demonstrate that they are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man.”
To refresh your memory, mesenchymal stem cells (MSCs) [Pronunciation] are multipotent adult stem cells, also called stromal stem cells, that can differentiate into many different types of cells within the human body, including bone, cartilage, muscle, neural, skin, and corneal cells. In humans, MSCs can be found in bone marrow, fat (“adipose tissue”), umbilical cord tissue (“Wharton’s Jelly”), or amniotic fluid (the fluid surrounding a fetus).
These days, Regenexx only offers Regenexx-C in the Cayman Islands. After flying to Grand Cayman, a patient’s bone marrow is aspirated much like a same-day procedure. What’s different is that the mesenchymal stem cells in bone marrow aspirate are grown to much greater numbers via lab culture expansion. These cultured cells can be injected during the same trip, about 10-12 days later, or cryopreserved for future use.
Most people will avail themselves of Regenexx-SD, since the company reportedly charges more than $20,000 for Regenexx-C, excluding travel and lodging costs. These two treatments provide a glimpse into the process required to produce a quality stem cell treatment.
Knee and Back Pain Trends
On any Sunday during American football season, the prone-to-failure human knee is on full display. It’s actually a miracle that given brutal opponent attacks and mind-bending lateral moves inherent to the sport, the knees of football players don’t give out more often.
With people around the world living longer and leading active lifestyles, more knees are succumbing to injury than in the past. A National Hospital Discharge Survey confirms that aging baby boomers are getting injured knees replaced at a greater rate, and at a younger age than ever before.
Between 2000 and 2010, more than 5.2 million total knee replacements (TKRs) were performed in the U.S. By 2010, TKRs were the leading inpatient surgery performed on adults aged 45-plus, with more than 700,000 performed each year.
The rate at which middle-aged and older American men and women had knees replaced almost doubled between 2000 and 2010, the researchers reported. And people aren’t putting off the procedure for as long, either. In 2000, the average knee replacement patient was about 69 years old, by 2010 that age had dropped to just over 66.
ACL tears among 14-18-year-olds have also skyrocketed, jumping 148% over the past decade, according to the American Academy of Pediatrics. The academy blames a variety of factors, including skeletal immaturity and decreased knee strength, most likely due to hours spent texting instead of exercising. 😂
The trend has gone global. In the Netherlands, about 29,000 patients underwent knee replacement surgery in 2017, according to the national registry of orthopedic implantations (LROI), a number that jumped 40% in eight years. De Volkskrant placed the blame squarely on Dutch people becoming “too fat at a younger age and staying active longer at an older age” (Dutch language link).
Then there is back pain. Some 16 million adults, 8% of U.S. adults, experience persistent or chronic back pain, and as a result, are limited in certain everyday activities. Back pain is the sixth most costly condition in the U.S., according to the Georgetown University Health Policy Institute, resulting in $12 billion per year in direct and indirect costs.
These statistics suggest that knee and lumbar pain would be ideal proxies for the overall effectiveness of stem-cell treatments, which led to the decision to create this resource.
Stem Cell Treatment for Knee Osteoarthritis?
This resource is more insightful due to the fact that yours truly had a partial meniscectomy in New York after injuring the left knee running after a grocery delivery boy. This exacerbated an injury that originally occurred while jogging in San Francisco a few years earlier.
The diagnostic imaging report of an MRI, edited to improve clarity, found:
“There is a truncation of the inner free edge of the body, anterior, and posterior horn of the medial meniscus over a 3.5 cm segment with [a] small, severely macerated remnant portion of the meniscus, suggesting prior arthroscopic partial medial meniscectomy. Medial compartment articular cartilage shows severe full-thickness cartilage loss [in the] central weight-bearing region [of the] medial femoral condyle (MFC) [and the] medial tibial plateau (MTP) with exposure of subchondral bone and moderate subchondral stress response. There are tricompartmental marginal osteophytes.”
A few definitions are in order:
Cartilage is the tough and flexible connective tissue found in many parts of the body that is relatively easy to damage. This rubbery tissue acts as a cushion between joint bones. Cartilage damage often results in joint pain, stiffness, and inflammation (swelling). That explains why cartilage loss observed in this MRI is a most worrisome finding because that means less cushioning between femur and tibia bones:
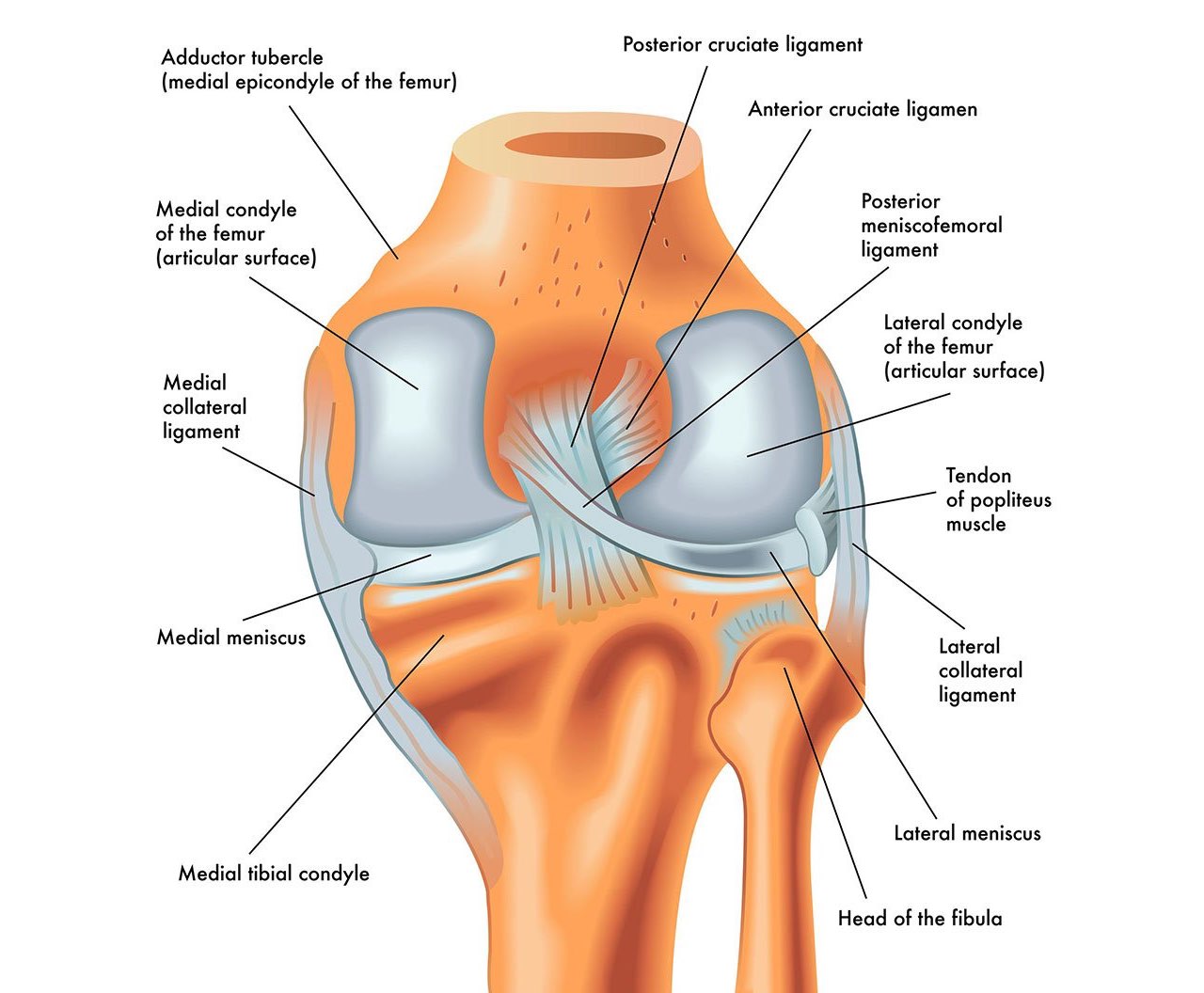 The knee is one of the largest and most complex joints in the body. The knee joins the thigh bone (femur) to the shin bone (tibia). The smaller bone that runs alongside the tibia (fibula) and the kneecap (patella) are the other bones that make the knee joint. Two C-shaped pieces of cartilage, called the medial and lateral menisci, act as shock absorbers between the femur and tibia.
The knee is one of the largest and most complex joints in the body. The knee joins the thigh bone (femur) to the shin bone (tibia). The smaller bone that runs alongside the tibia (fibula) and the kneecap (patella) are the other bones that make the knee joint. Two C-shaped pieces of cartilage, called the medial and lateral menisci, act as shock absorbers between the femur and tibia.
Subchondral bone sits underneath cartilage in large joints, such as knees and hips, as well as in small joints found in hands and feet.
Marginal osteophytes are a common occurrence in osteoarthritis of the knee. These osseous (“bone-like”) outgrowths are formed at the junction between cartilage and bone.
Osteoarthritis (OA) is a condition in which cartilage deteriorates over time, which can lead to “bone on bone” inflammation — the severest form of osteoarthritis. There is strong evidence that a meniscectomy increases the likelihood of osteoarthritis. More than 30 million people in the U.S. suffer from osteoarthritis, including 14 million with bad knees.
Stem-cell therapy may also increase the cartilage volume, however, the unpredictability of results keeps many sufferers sidelined. While many studies suggest that stem cell therapy has a good chance of restoring some prior function of a knee afflicted with OA, it’s best to lower your expectations when it comes to “severe medial compartment osteoarthritis,” as the radiologist, Dr. Joseph Wall, noted in his MRI radiology report.
Stem Cell Treatment vs. Knee Replacement
The most often recommended treatments for severe knee OA are either a total knee replacement (TKR), also known as total knee arthroplasty (TKA), or a partial knee replacement (PKR) — an expensive and invasive surgical procedure that introduces its own risks.
One research study reports that “TKA is a successful treatment for osteoarthritis of the knee with an expectable revision rate of less than 5% within 10 years and a long-lasting functional improvement of more than 30% in any assessment score.”
That low revision surgery rate is dependent on the quality of care one receives, including how skilled the orthopedic surgeon is; if a knee surgery robot is employed; and what TKR replacement techniques are used:
- Surgeon expertise – An orthopedic surgeon with extensive knee surgery experience will increase the chance of procedure success. UK registry data suggests that low-volume surgeons have a higher revision rate.
- Knee surgery robots – While several studies report that knee surgery robots offer better alignment accuracy and implant fit compared to a manual TKR, none have equated that performance to an improvement in clinical outcomes.
- Surgical techniques – Cementless fixation, which eliminates the polymethyl methacrylate cement typically used to attach knee implants promises to shorten operation time, with the expectation that this technique could reduce rates of implant wear and loosening. Another technique to look for is a minimally stabilized TKR with cross-linked polyethylene bearing type, which, according to an Australian study, outperformed a posterior stabilized TKR with non-cross-linked polyethylene which increases the risk of infections by 102%.
Philippe Hernigou released several studies in recent years, including one that summarized the findings of 140 adults aged 65 to 90 years who had been scheduled for bilateral (both knees) total knee replacement (TKR or TKA) and instead received one TKA in one knee and BMAC in the other. The study found:
At the most recent follow up (average of 15 years, range 10 to 20 years), a total of 25 (18%) of the 140 patients underwent total knee arthroplasty performed at a mean of ten years (range, 5 to 15 years) after the date of the cell therapy. This study showed that subchondral bone marrow concentrate (as compared with TKA) had a sufficient effect on pain to postpone or avoid the TKA in the contra lateral joint of patients with bilateral osteoarthritis.
In a 2018 study, Hernigou evaluated 30 patients who had bilateral knee osteoarthritis with severe joint space narrowing, and who received TKA in one knee and subchondral (injection in the bone itself) bone marrow concentrate injection in the contralateral (other) knee. This sample was much younger with an average age of 28 years and a range of 18-41:
The Knee Score had improved and remained similar in the TKA and cell therapy groups (respectively 80.3 points ± 11 versus 78.3 ± 23); 21 patients preferred the knee with cell therapy and nine preferred the knee with TKA. Knees with cell therapy registered an improvement in cartilage and bone marrow lesions, observed at the site of bone marrow subchondral injection using MRI.
The biggest risk of a TKR is revision surgery, due either to the loosening or bad fit of an implant, or an infection. Christopher Vertullo’s Australian study, cited earlier, notes that “infection of total knee replacements (TKRs) remains concerningly frequent, [and are] devastating for patients.”
If you are one out of 20 patients who needs revision surgery, you could be at risk for much financial exposure. TKR revision surgery is complex and costs upward of $74,000 on average in the U.S.
Then there is the frustrating possibility of continued knee pain after knee replacement surgery, which, while rare, is a known issue.
After considering all these factors, a leap of faith in a stem-cell therapy that might stave off an invasive TKR operation for one to two years was indicated, as a physician might put it.
What follows next is Part II of this well-documented journey into orthobiologics with many, hopefully, useful observations for prospective patients.
Read next: Part II. Why Have a Stem Cell Treatment?
